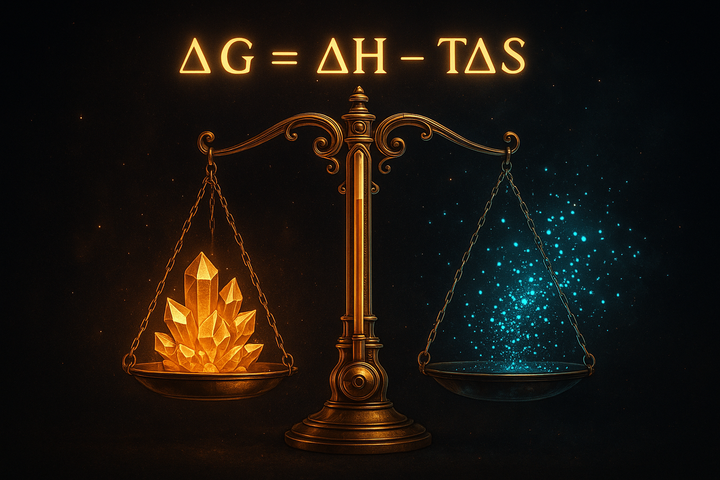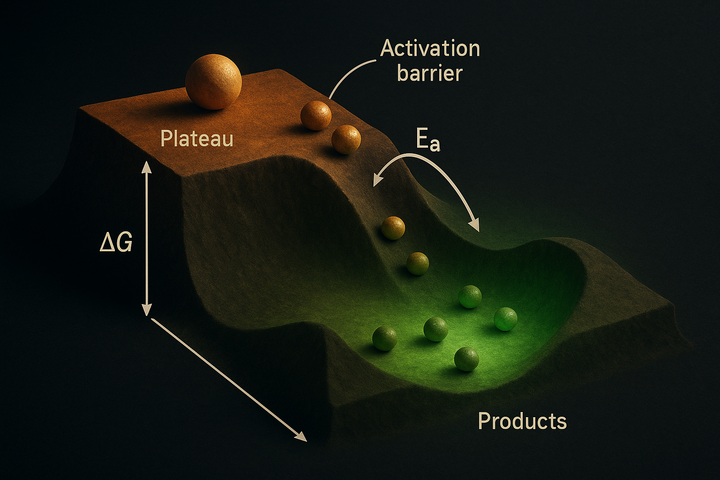
Spontaneous Reactions: When ΔG Goes Negative
The word "spontaneous" misleads everyone.
In everyday language, spontaneous means sudden, impulsive, happening immediately. A spontaneous decision. A spontaneous combustion.
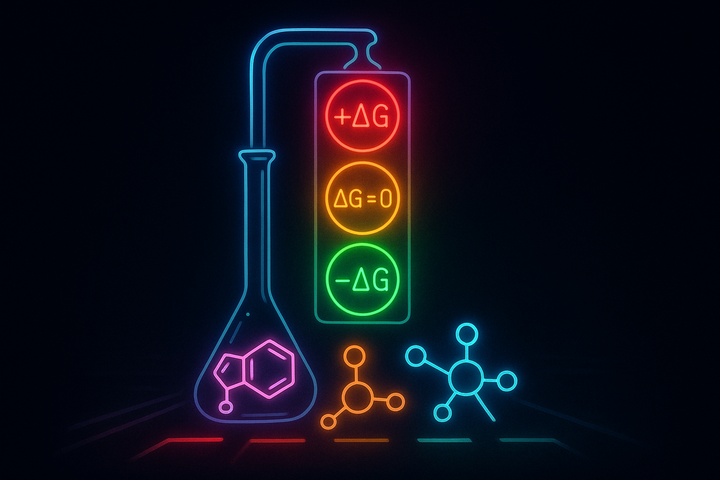
In thermodynamics, spontaneous means something precise and different: thermodynamically favorable. A spontaneous reaction is one where ΔG < 0. That's it. No claim about speed, no