Cochlear Implants: The Success Story
If you want to know what a mature neural interface technology looks like—not experimental, not research-only, but actually working in the real world at scale—look at cochlear implants.
Over a million people worldwide have them. Children born deaf get implanted before their first birthday and grow up hearing. Adults who lost their hearing regain enough to have phone conversations, listen to music, and navigate a hearing world. The technology was first approved in the 1980s and has been refined for forty years.
This is the proof of concept. This is what neural interfaces can become.
And almost nobody thinks of cochlear implants as "cyborg technology." They're just... medicine. Routine. Boring in the best way.
The endgame for brain-computer interfaces is to be this boring. To be so normal that nobody notices.
How Hearing Works (and Fails)
To understand cochlear implants, you need to understand how normal hearing works—and where it can break.
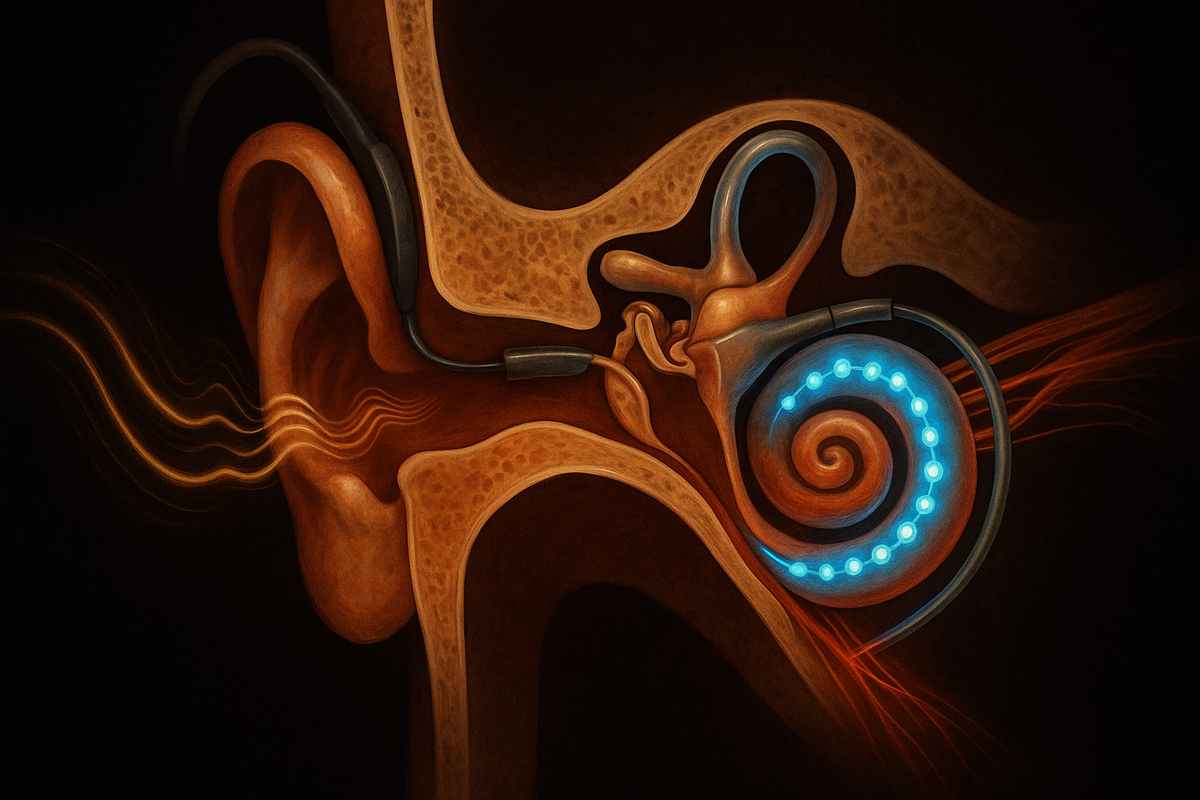
Sound waves enter the ear canal and vibrate the eardrum. The vibrations pass through tiny bones (the ossicles) to the cochlea—a snail-shaped, fluid-filled structure in the inner ear. Inside the cochlea are hair cells: specialized sensory cells that convert the mechanical vibrations into electrical signals. These signals travel via the auditory nerve to the brain, where they're processed as sound.
Most hearing loss happens because the hair cells are damaged or destroyed. Loud noise, aging, genetics, infection, certain medications—many things can kill hair cells. And unlike many cells in your body, hair cells don't regenerate. Once they're gone, they're gone.
Here's the key insight: in most cases of profound hearing loss, the auditory nerve is still intact. The cells that convert vibration to electrical signal are dead, but the nerve that carries electrical signal to the brain still works.
A cochlear implant replaces the hair cells. It converts sound directly to electrical stimulation of the auditory nerve, bypassing the damaged cochlea entirely.
The bridge is out. We built a new one.
The Device
A cochlear implant has two parts: external and internal.
The external part looks like a behind-the-ear hearing aid. It contains a microphone, a processor, and a transmitter. The microphone picks up sound. The processor converts the sound into a pattern of electrical signals—essentially, the code that will stimulate the nerve. The transmitter sends this signal across the skin to the internal part, using radio waves and magnetic coupling.
The internal part is surgically implanted. A receiver sits under the skin behind the ear, held in place by the transmitter's magnet. A wire leads from the receiver into the cochlea, where an array of electrodes coils along its length. When the receiver gets signals from the transmitter, it delivers electrical current through specific electrodes, stimulating the auditory nerve fibers at specific locations.
Different locations along the cochlea naturally respond to different sound frequencies—high frequencies near the base, low frequencies near the apex. The implant electrode array mimics this: high-frequency sounds activate electrodes near the base, low-frequency sounds activate electrodes near the apex. The brain receives a pattern of stimulation that maps to the frequency content of the original sound.
This is the fundamental trick: converting acoustic information into electrical patterns that the auditory nerve can carry to the brain.
What It Sounds Like
Here's what people don't always understand: cochlear implants don't restore normal hearing. They provide a substitute that the brain learns to interpret.
Early cochlear implant recipients often describe the sound as robotic, mechanical, or chipmunk-like. Voices don't sound quite like voices. Music is strange. Environmental sounds are confusing.
But the brain adapts. Over weeks and months, the strange signals become interpretable. Recipients learn to understand speech, often remarkably well. Many can have phone conversations, watch TV without captions, and navigate noisy environments.
Music is harder. Cochlear implants have limited frequency resolution compared to natural hearing—typically 12-22 electrode channels versus the roughly 3,500 hair cells in a normal cochlea. This means the pitch information is crude. Melodies are recognizable but don't sound quite right. Some recipients enjoy music despite the limitations; others find it frustrating.
Children implanted early—before age 2 or 3—often do remarkably well. Their brains are plastic enough to learn the implant's signals as their native hearing. They develop speech and language at near-normal rates. By school age, many are indistinguishable from their hearing peers.
Adults implanted later in life typically don't reach the same level of performance. Their brains already have a template for what speech should sound like, and the implant signals don't match. But most still gain enough hearing to significantly improve their lives.
Cochlear implants don't give you normal hearing. They give you enough.
The History
The idea of electrical hearing dates back to Alessandro Volta in 1800, who stuck electrodes in his ears and reported hearing noise when he passed current through them. (Don't try this.)
Serious development began in the 1950s and 1960s, with researchers demonstrating that electrical stimulation of the auditory nerve could produce sound percepts. By the 1970s, several groups were developing multi-electrode implants.
The first FDA-approved cochlear implant was the House/3M device in 1984. It had just one electrode channel and provided limited benefit—mostly awareness of environmental sounds rather than speech understanding. But it proved the concept: electrical stimulation could provide meaningful hearing.
Multi-channel devices followed in the late 1980s and 1990s. Cochlear Ltd. (Australia), Advanced Bionics (US), and MED-EL (Austria) became the major manufacturers. Each generation of devices improved on the last: more electrodes, better signal processing, smaller external components, longer battery life.
By the 2000s, cochlear implants had become standard of care for severe-to-profound hearing loss when hearing aids weren't sufficient. Insurance typically covers them. Thousands of surgeons worldwide perform the procedure. The waiting lists are for surgery scheduling, not research enrollment.
Forty years from "interesting experiment" to "routine medicine." That's the timeline for neural interfaces.
The Numbers
Let's get specific about what cochlear implants achieve.
Speech understanding in quiet: Many cochlear implant users score 80-90% on sentence recognition tests in quiet listening conditions. Some achieve near-perfect scores.
Speech understanding in noise: This is harder. Background noise is challenging for cochlear implant users because the limited frequency resolution makes it hard to separate the target voice from competing sounds. But many users still function well in moderately noisy environments.
Language development in children: Kids implanted before age 2 often reach language milestones at near-normal ages. Kids implanted later can catch up significantly but may have persistent gaps.
Music appreciation: Mixed results. Many users enjoy music despite its altered quality. Some former musicians find it frustrating. Pitch perception remains a weakness of current devices.
Quality of life: Studies consistently show large improvements in quality of life, social participation, and educational/employment outcomes for cochlear implant recipients.
These numbers don't capture everything. Cochlear implants can be exhausting—processing degraded sound takes cognitive effort. They don't work in water. They need external components that can be lost or damaged. They require follow-up appointments and adjustments.
But for most recipients, the benefits vastly outweigh the limitations.
The Deaf Community Debate
Cochlear implants have been controversial within the Deaf community—and that controversy matters for how we think about neural interfaces more broadly.
Some Deaf advocates view cochlear implants as an attack on Deaf culture and identity. They argue that deafness is not a medical problem to be fixed but a cultural difference to be accommodated. Implanting deaf children, in this view, is a form of cultural erasure—denying them membership in the Deaf community and forcing them into a hearing world that may never fully accept them.
Others, including many deaf individuals and parents of deaf children, see cochlear implants as a valuable option that expands possibilities. Having access to spoken language doesn't preclude also learning sign language. The implant is a tool, not a verdict on identity.
This debate isn't just philosophical. It has real implications for:
Parental decision-making. Most deaf children are born to hearing parents who face an agonizing choice: implant early for best language outcomes, or wait and potentially limit the child's options.
Resource allocation. Money spent on cochlear implants could alternatively support Deaf education, sign language interpreters, and accessibility accommodations.
Identity and belonging. Implanted individuals sometimes struggle to fit in either hearing or Deaf communities—too deaf for one, too hearing for the other.
There's no single right answer. The debate highlights a truth that will recur as neural interfaces advance: these technologies don't just restore function. They change identity, community, and culture.
Fixing "disability" is never neutral. It implies a judgment about what needed fixing.
What Cochlear Implants Teach Us
Cochlear implants are a model for other neural interfaces. Here's what their history teaches:
Brain plasticity is key. The auditory system adapts to the implant's signals over time. Children adapt more fully than adults. This suggests that motor BCIs, sensory BCIs, and other interfaces will also depend on the brain's ability to learn.
Early implantation matters. For language development, earlier is better. This may or may not generalize to other interfaces—it depends on the specific developmental windows for each brain system.
Good enough is good enough. Cochlear implants don't restore normal hearing. They provide a functional substitute. Future neural interfaces probably won't restore normal function either—but they might provide something valuable nonetheless.
Technology improves over time. Early cochlear implants were crude. Current devices are sophisticated. Recipients from the 1980s have benefited from upgrades. Future BCIs will similarly improve through iteration.
Social integration matters as much as technical performance. Cochlear implant recipients don't just need the technology to work—they need schools that accommodate them, workplaces that support them, and social contexts that include them. Technology alone is not sufficient.
Controversy is persistent. After forty years, cochlear implants remain controversial in some circles. Future neural interfaces will face similar debates, probably more intense.
The Future of Auditory Interfaces
Cochlear implants are mature technology, but they're still improving.
Higher electrode counts. Current implants have 12-22 electrodes. Research is exploring denser arrays that might provide finer frequency resolution and better music perception.
Hybrid devices. Some recipients have residual low-frequency hearing that cochlear implants damage during insertion. Hybrid devices preserve this low-frequency hearing while adding electrical stimulation for high frequencies—the best of both worlds.
Fully implanted systems. Current devices require external processors. Fully implanted systems (with internal microphones and processors) would be more convenient and more robust—no visible components, no risk of loss.
Auditory brainstem implants. For patients whose auditory nerves are damaged (not just their cochleae), implants can target the brainstem directly. Results are currently worse than cochlear implants, but the technology is advancing.
Central auditory implants. The ultimate step: implanting the auditory cortex directly. This is speculative but not impossible—if we can stimulate sensory cortex for touch, why not for sound?
The Bigger Picture
Cochlear implants demonstrate that neural interfaces can move from research to routine medicine. The path took decades, required massive investment, and involved countless patients willing to be early adopters.
But it worked. A million people can hear who otherwise couldn't. Children learn language. Adults stay connected. Lives are transformed at scale.
This is what success looks like. Not flashy demonstrations or science fiction promises. Just steady improvement, broad adoption, and meaningful improvement in lives.
Motor BCIs, sensory BCIs, and whatever comes next are on the same path. Further back. Earlier stage. More experimental. But the destination is visible: a world where neural interfaces are as unremarkable as glasses or pacemakers.
Cochlear implants are proof that neural interfaces can become boring.
And boring, in this context, is the highest achievement. It means the technology works so well that nobody thinks about it anymore. They just use it and get on with their lives.
That's the future we're building toward.
The Personal Dimension
Let me tell you about what activation day looks like.
A cochlear implant is surgically implanted, but it isn't turned on immediately. The surgical site needs to heal—typically 2-4 weeks. During this time, the recipient is still deaf. They have a device in their head that doesn't do anything yet.
Then comes activation. The audiologist connects the external processor for the first time. Electrodes are tested one by one. Levels are adjusted. And then—sound.
There are countless videos online of this moment. A baby hearing her mother's voice for the first time, bursting into tears. A teenager hearing music after years of silence. An elderly man hearing his grandchildren speak.
The reactions vary. Some people cry with joy. Some are confused by the strange new sensations. Some don't react much at first—it takes time for the brain to make sense of the signals. But almost universally, there's a moment of recognition: I can hear something.
It's not magic. The sound is crude at first. Speech often sounds like robots or chipmunks. It takes weeks or months of practice for the brain to learn to interpret the signals. Some people never achieve great speech understanding.
But that moment of activation—when sound returns after silence—is something profound. It's the moment when a neural interface begins to function. When a piece of technology becomes part of a person.
Every cochlear implant activation is a proof of concept for the entire field of neural interfaces.
The Unsung Miracle
Here's what strikes me about cochlear implants: they're almost too successful to be interesting.
They've worked so well, for so long, that we've stopped thinking of them as remarkable. A deaf child gets an implant, learns to hear, goes to regular school, grows up essentially normally. That's not news. That's Tuesday.
But step back and see it clearly. We are directly stimulating the human nervous system to create sensory experience. We are bypassing damaged biology with engineered electronics. We are writing information into the brain.
This is science fiction made mundane through repetition. This is the cyborg future, already here, just not evenly distributed.
Over a million people are walking around with computers in their heads that translate sound into neural signals. And most of them are just... living their lives. Going to work. Raising kids. Not thinking about how extraordinary their situation is.
The most successful neural interface technology is the one you forget you're using.
Cochlear implants have achieved that. They've become invisible in their success. They're not a topic of excited speculation or philosophical debate. They're just... hearing aids that require surgery.
That invisibility is the goal for all neural interfaces. And cochlear implants prove it's achievable.
Looking Forward
The next generation of neural interfaces will build on what cochlear implants demonstrated:
That the brain can learn to interpret artificial signals as natural sensation. Touch, vision, proprioception—all should be teachable, given the right signals and enough time for adaptation.
That device reliability at scale is achievable. A million implants working for decades proves that we can make electronics that survive in the body.
That insurance and healthcare systems can incorporate neural interfaces. The path from experimental to routine is navigable, even if slow.
That people can live normal lives with computers in their heads. The social and psychological integration is possible.
Motor BCIs, visual prosthetics, and bidirectional interfaces are all earlier on this path. But the destination is proven possible.
Cochlear implants are not just a success story. They're a roadmap.
Follow it forward, and you see a future where neural interfaces of many kinds become as unremarkable as cochlear implants are today. Where paralyzed people controlling computers is boring. Where artificial touch is routine. Where the line between biological and technological is just... not something we think about much anymore.
That future is decades away. But cochlear implants show it's not fantasy.
One million people are already there.



Comments ()