The Gibbs Free Energy Equation
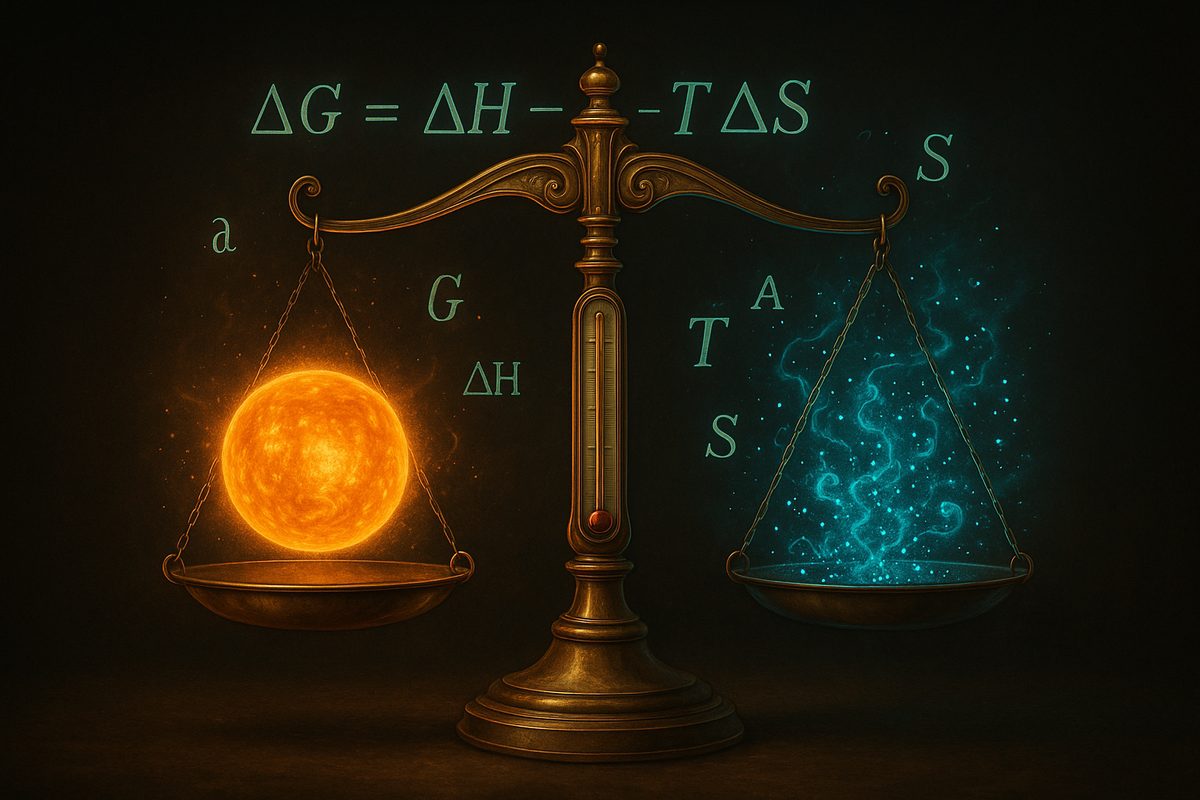
The Universe's Decision Function
Here's something that should stop you in your tracks: the universe has an optimization criterion.
It's not conscious. It doesn't deliberate. But every chemical reaction, every phase transition, every biological process happening in your body right now is being evaluated by a single quantity. And that quantity decides—with mathematical precision—whether something will happen spontaneously or not.
Gibbs free energy.
When chemists want to know if a reaction will proceed on its own, they don't guess. They calculate ΔG. When your cells decide whether to burn glucose or store it, they're following ΔG. When ice melts at exactly 0°C and not 1°C below or above, it's because ΔG crosses zero at that temperature.
ΔG = ΔH - TΔS
That's the equation. Enthalpy minus temperature times entropy. Three quantities that capture the universe's accounting for energy and disorder. The sign of ΔG decides everything: negative means the reaction runs forward spontaneously. Positive means it requires input. Zero means equilibrium—the system is balanced and going nowhere.
This is the most powerful equation in thermodynamics.
The Core Insight
Gibbs free energy is what's left over after paying the entropy tax.
Energy wants to spread out. Disorder wants to increase. Every process is caught between these two demands. The Gibbs equation captures the competition: ΔH measures the energy change, TΔS measures the entropy contribution, and ΔG tells you which one wins.
At low temperatures, energy dominates. Reactions that release heat (negative ΔH) win. At high temperatures, entropy dominates. Reactions that increase disorder (positive ΔS) win. The crossover point—where ΔG equals zero—is where phase transitions happen.
This is why ice melts at a specific temperature. This is why proteins fold into specific shapes. This is why life requires constant energy input to maintain its improbable organization. The Gibbs equation is the accounting ledger for everything that happens.
The Series
This series explores how one equation becomes the universe's decision function.
Gibbs Free Energy Explained — What ΔG actually measures and why it matters. The connection to spontaneity and equilibrium.
The Formula: ΔG = ΔH - TΔS — Decoding the equation. What each term contributes and how they interact.
What Is Delta G? — The sign that decides everything. Negative, positive, zero—and what each means.
Spontaneous Reactions — When ΔG goes negative. What "spontaneous" actually means (hint: it's not about speed).
The Gibbs-Helmholtz Equation — Temperature's hidden power. How heating changes which reactions are favorable.
Chemical Equilibrium — Where ΔG equals zero. Why equilibrium isn't static and what the equilibrium constant tells you.
ATP Hydrolysis — The -30.5 kJ/mol that runs your body. Why ATP is the universal energy currency.
Protein Folding — Finding the free energy minimum. How proteins navigate a landscape with 10^300 possible configurations.
Phase Transitions — Why ice melts at exactly 0°C. How ΔG explains solid, liquid, and gas.
Diamonds and Graphite — Thermodynamics says diamonds should turn to graphite. Kinetics says wait. The difference between favorable and fast.
Membrane Potential — Free energy across barriers. How cells maintain gradients that power everything they do.
Synthesis: Free Energy as the Logic of Becoming — What ΔG reveals about change, possibility, and why the universe has a direction.
Why This Matters
Every time you ask "will this reaction happen?" you're asking a ΔG question. Every time a cell decides to do anything, it's consulting ΔG. Every time matter changes state—ice to water, water to steam, solid to solution—ΔG is crossing zero.
The Gibbs equation isn't just chemistry. It's the universe's decision procedure.
Every system minimizes its free energy. Every stable state is a free energy minimum. Every transition is the universe finding a lower-energy configuration that also maximizes what can be randomized.
This isn't metaphor. It's physics. And once you see it, you see it everywhere.
Start with Gibbs Free Energy Explained to understand the quantity that decides whether reactions happen.



Comments ()